Anoda berbasis silikon memiliki kapasitas spesifik teoritis sebesar 4200 mAh/g. Kapasitas ini jauh melebihi kapasitas anoda grafit tradisional yang hanya 372 mAh/g. Hal ini telah menjadi kunci utama untuk mengatasi hambatan kinerja anoda konvensional. Teknologi deposisi uap kimia (CVD) memungkinkan deposisi silikon yang seragam pada substrat karbon. Teknologi ini juga menciptakan antarmuka silikon-karbon yang stabil. Proses ini telah menjadi jalur utama untuk komersialisasi anoda silikon-karbon. Dalam proses ini, bahan karbon berpori bukan sekadar "pembawa". Mereka adalah "perancah inti" yang menentukan batas kinerja anoda silikon-karbon CVD. Kinerja mereka secara langsung memengaruhi kinerja elektrokimia dan kelayakan komersialisasi material komposit.
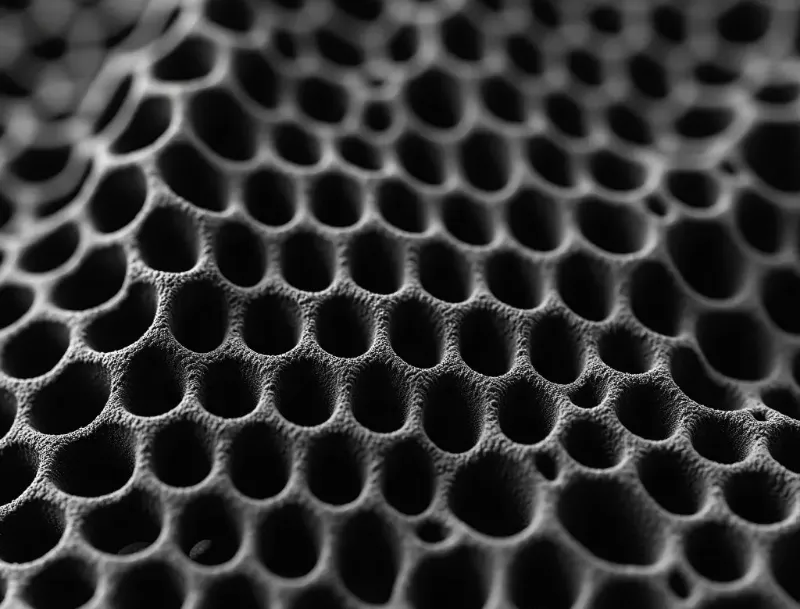
Pemahaman Dasar tentang Karbon Berpori
Karbon berpori adalah material berbasis karbon dengan pori-pori yang saling terhubung. Menurut standar IUPAC, pori-pori diklasifikasikan sebagai mikropori (<2 nm), mesopori (2-50 nm), dan makropori (>50 nm). Karakteristik struktural dari berbagai ukuran pori menentukan fungsinya dalam anoda silikon-karbon CVD.
Karbon berpori juga memiliki resistansi listrik yang rendah. Karbon ini tahan terhadap suhu tinggi, asam, dan basa. Karbon berpori dapat membangun jaringan konduktif yang stabil, memastikan masa pakai siklus elektroda. Rasio pori dan porositas dapat disesuaikan selama proses untuk memenuhi berbagai persyaratan kinerja anoda silikon-karbon CVD.
Peran Inti dan Keuntungan Karbon Berpori dalam Anoda Silikon-Karbon CVD
Saat ini, industrialisasi anoda silikon-karbon CVD menghadapi dua tantangan utama:
Ekspansi volume silikon selama pemasangan litium menyebabkan penghancuran elektroda dan pelepasan bahan aktif.
Reaksi samping antara silikon dan elektrolit menghasilkan lapisan SEI tebal, yang mengurangi efisiensi siklus pertama dan masa pakai siklus baterai.
Karbon berpori menawarkan solusi untuk dua masalah ini:
Menyangga ekspansi volume silikon:
Struktur pori bertingkat pada karbon berpori membentuk "sistem penyangga tiga tingkat". Pori-pori ini menyediakan ruang fisik bagi silikon untuk mengembang. Pori-pori ini juga menyebarkan tekanan melalui deformasi elastis, sehingga mengurangi risiko fraktur partikel. Mesopori menyesuaikan ukuran partikel silikon. Setelah penyisipan litium, silikon mengembang dan mengisi pori-pori ini, mencegah kompresi timbal balik antar partikel.
Mengisolasi silikon dari elektrolit dan menstabilkan lapisan SEI:
Di satu sisi, kerangka karbon karbon berpori membungkus nanopartikel silikon. Hal ini mengurangi kontak langsung antara silikon dan elektrolit. CVD juga melibatkan pelapisan karbon sekunder. Hal ini membentuk lapisan karbon padat pada permukaan material komposit karbon/silikon berpori. Isolasi ganda mengurangi reaksi samping lebih dari 60%. Di sisi lain, reaksi samping yang berkurang mencegah pembentukan dan pelepasan lapisan SEI akibat fraktur partikel silikon. Hal ini meningkatkan efisiensi konversi energi dan siklus hidup.

Metode Persiapan Karbon Berpori
Metode Aktivasi
Metode aktivasi melibatkan pencampuran prekursor karbon dengan agen pengaktif dan melakukan reaksi pembentukan pori dalam kondisi gas inert bersuhu tinggi. Metode ini mencakup aktivasi fisik dan aktivasi kimia.
(1) Aktivasi fisik:
Material berbasis biomassa atau batu bara seperti tempurung kelapa atau antrasit digunakan sebagai prekursor. Setelah dihancurkan dan dihilangkan pengotornya, material tersebut dikarbonisasi pada suhu tinggi untuk membentuk kerangka karbon awal. CO₂ atau uap kemudian ditambahkan sebagai agen pengaktif pada suhu 800-1100°C untuk mengetsa kerangka karbon dan membentuk pori-pori. Setelah pendinginan dan penyaringan, produk siap digunakan. Metode ini ramah lingkungan, bebas residu reagen kimia, berbiaya rendah, dan cocok untuk kelas menengah ke bawah. produksi karbon berporiNamun, kandungan mesopori umumnya terbatas di bawah 50%, yang mungkin tidak memenuhi persyaratan beban silikon tinggi.
(2) Aktivasi kimia:
Material berkarbon tinggi, seperti resin fenolik atau antrasit, digunakan sebagai prekursor. Prekursor dicampur dengan zat pengaktif dengan rasio 3:1. Kemudian, prekursor dipanaskan untuk karbonisasi dan aktivasi. Setelah reaksi, zat pengaktif dicuci, dan material dikeringkan. Karbon berpori yang dibuat menggunakan aktivasi kimia memiliki kandungan mesopori yang lebih tinggi, kontrol struktur pori yang lebih kuat, dan luas permukaan hingga 2500-3000 m²/g.
Metode Template

Dalam metode templat, prekursor karbon dimasukkan ke dalam templat dan dipanaskan pada suhu tinggi. Prekursor tersebut secara bertahap mengalami karbonisasi, dan templat kemudian dihilangkan untuk menghasilkan karbon berpori. Metode ini dibagi menjadi metode templat keras dan templat lunak.
(1) Metode templat keras:
Material seperti alumina atau saringan molekuler dengan struktur pori tetap digunakan sebagai templat. Prekursor diimpregnasi ke dalam pori-pori templat. Setelah karbonisasi pada suhu 800-1000°C, templat dilarutkan menggunakan asam untuk menghasilkan karbon berpori dengan struktur pori komplementer. Metode ini menghasilkan orde mesopori lebih besar dari 90% dan deviasi ukuran pori <5%. Metode ini memastikan deposisi silikon yang seragam, tetapi biaya templatnya tinggi dan prosesnya rumit. Metode ini digunakan untuk penelitian laboratorium atau produksi skala kecil dan canggih.
(2) Metode templat lunak:
Kopolimer blok atau surfaktan digunakan sebagai templat. Mereka merakit diri menjadi misel mesopori ketika dicampur dengan prekursor karbon seperti sukrosa atau resin fenolik. Campuran tersebut kemudian dikarbonisasi pada suhu 600-800°C. Metode ini menghasilkan mesopori yang terdiri dari 60-70% struktur, dengan biaya yang lebih rendah daripada metode templat keras.
Metode Sol-Gel
Metode sol-gel melibatkan pencampuran garam alkohol atau garam logam anorganik dengan pelarut untuk membentuk larutan, yang kemudian mengalami hidrolisis dan kondensasi untuk membentuk sol-gel. Setelah proses penuaan, pengeringan, dan sintering suhu rendah, karbon berpori dihasilkan. Dalam sintesis sol-gel, keruntuhan pori dapat terjadi selama tahap pengeringan. Untuk menghindari hal ini, metode templat sering digunakan bersamaan dengan metode sol-gel.
Bubuk Epik
Epic Powder memiliki pengalaman lebih dari 20 tahun dalam pemrosesan bubuk. Kami menawarkan solusi terintegrasi mulai dari penghancuran, penggilingan, klasifikasi ke modifikasiDengan mengoptimalkan penyiapan material karbon berpori, kami memastikan peningkatan kinerja dan siklus jangka panjang untuk anoda silikon-karbon CVD, yang berkontribusi pada komersialisasi baterai litium-ion berkinerja tinggi.

